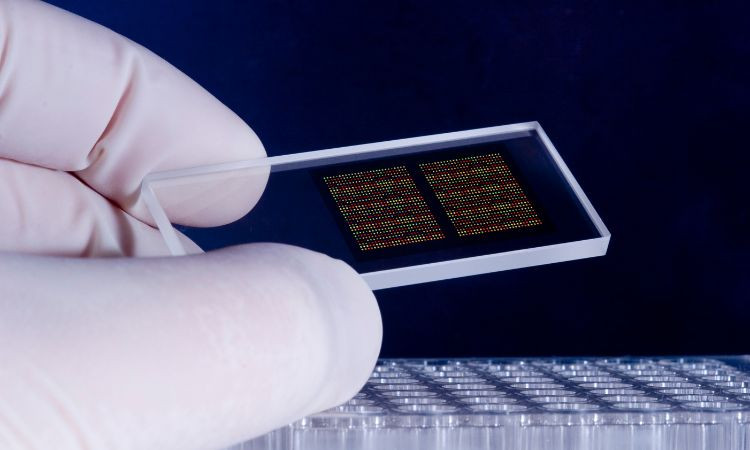
The field of genomics has witnessed remarkable growth over the past few decades, driven by technological advancements that have revolutionized our understanding of the genetic basis of life. One such technology that has played a pivotal role in genomics research is DNA microarray technology. The global DNA microarray market size attained a value of nearly USD 5.14 billion in 2023. The market is further estimated to grow in the forecast period of 2024-2032 at a CAGR of 10.6% to reach about USD 12.69 billion by 2032. This significant growth underscores the critical importance of DNA microarray technology in various fields, from basic research to clinical diagnostics and personalized medicine. In this blog post, we will delve into the fascinating journey of DNA microarray technology, tracing its origins, examining its present state, and envisioning its future innovations.
I. Historical Background
To understand the evolution of DNA microarray technology, it is essential to explore its historical roots. The origins of DNA microarrays can be traced back to the early developments in molecular biology, particularly in techniques such as Southern blotting and hybridization. Southern blotting, developed in the 1970s by Edwin Southern, revolutionized the field by allowing researchers to detect specific DNA sequences within complex mixtures. This technique laid the groundwork for subsequent advancements in DNA analysis.
In the 1980s and 1990s, researchers began exploring the possibility of miniaturizing DNA analysis techniques to enable high-throughput analysis of gene expression. This led to the invention of the first DNA microarrays, which allowed researchers to simultaneously analyze thousands of genes on a single chip. One of the earliest microarray technologies, known as the DNA chip, was developed by Stephen Fodor and colleagues at Affymetrix in the early 1990s. This groundbreaking technology paved the way for the widespread adoption of microarray technology in genomics research.
II. Present State of DNA Microarrays
Today, DNA microarrays come in various forms, including oligonucleotide arrays, cDNA arrays, and SNP arrays, each with its unique applications. Oligonucleotide arrays, for example, consist of short DNA probes that are synthesized directly onto a solid substrate, while cDNA arrays utilize complementary DNA (cDNA) probes generated from mRNA samples. SNP arrays, on the other hand, are designed to detect single nucleotide polymorphisms (SNPs) within the genome.
The present state of DNA microarrays is characterized by their widespread adoption in both academic and industrial settings. Major companies and research institutions drive innovation in this field, contributing to its rapid growth and expansion. The applications of DNA microarrays are diverse, ranging from gene expression profiling and genome-wide association studies to drug discovery and disease diagnosis.
In gene expression profiling, DNA microarrays are used to measure the expression levels of thousands of genes simultaneously, providing valuable insights into the molecular mechanisms underlying various biological processes. Genome-wide association studies (GWAS) utilize DNA microarrays to identify genetic variants associated with complex diseases and traits, helping to unravel the genetic basis of common disorders such as cancer, diabetes, and cardiovascular disease.
In drug discovery, DNA microarrays are used to screen for potential drug targets and identify biomarkers that can predict drug response and toxicity. In disease diagnosis, DNA microarrays are used to detect genetic mutations and gene expression patterns associated with specific diseases, enabling early detection and personalized treatment strategies.
III. Challenges and Limitations
Despite their widespread adoption, DNA microarrays face several challenges that must be addressed to fully realize their potential. One of the major challenges is related to data quality and reproducibility. Variability in sample preparation, hybridization conditions, and data analysis methods can lead to inconsistencies in results, making it difficult to compare data across different studies.
Standardization of protocols is another challenge facing the field of DNA microarrays. There is a lack of consensus on best practices for sample preparation, hybridization, and data analysis, leading to variability in results and hindering the reproducibility of studies.
Ethical and regulatory considerations also pose significant challenges to the widespread adoption of DNA microarray technology. Privacy concerns related to genetic testing and the use of personal genomic data raise important ethical questions that must be addressed. Regulatory oversight and compliance are also important considerations, particularly in the context of clinical diagnostics and personalized medicine.
IV. Future Directions and Emerging Trends
Looking ahead, DNA microarray technology is poised for continued innovation and advancement. Next-generation sequencing integration, single-cell analysis, and spatial transcriptomics are among the emerging trends shaping the future of DNA microarrays.
Next-generation sequencing (NGS) technologies, such as RNA sequencing (RNA-seq) and whole-genome sequencing (WGS), are increasingly being integrated with DNA microarrays to provide complementary information about gene expression, genetic variation, and epigenetic modifications. This integrated approach enables researchers to gain a more comprehensive understanding of complex biological processes and disease mechanisms.
Single-cell analysis is another emerging trend in genomics research, driven by recent advances in microfluidics and single-cell sequencing technologies. DNA microarrays are being adapted to analyze gene expression at the single-cell level, allowing researchers to explore cellular heterogeneity and identify rare cell populations that may play a role in disease progression and treatment response.
Spatial transcriptomics is also gaining momentum as a powerful tool for studying gene expression patterns within the context of tissue architecture. By spatially mapping gene expression profiles onto tissue sections, researchers can gain insights into the spatial organization of cells and their interactions within the tissue microenvironment.